Updated By: LatestGKGS Desk
Planck constant Definition, Unit, Symbol, Formula, Features, Unit for Measurement
Planck constant - Basic Unit for Measurement (Time, Length and Mass) Definition, Unit, Symbol, Formula
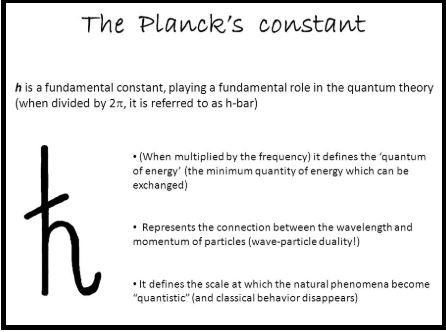
Planck constant, (symbol h), fundamental physical constant characteristic of the mathematical formulations of quantum mechanics, which describes the behavior of particles and waves on the atomic scale, including the particle aspect of light.
The German physicist Max Planck introduced the constant in 1900 in his accurate formulation of the distribution of the radiation emitted by a blackbody, or perfect absorber of radiant energy (see Planck’s radiation law).
The significance of Planck constant in this context is that radiation, such as light, is emitted, transmitted, and absorbed in discrete energy packets, or quanta, determined by the frequency of the radiation and the value of Planck’s constant.
In the International System of units (SI), the constant is equal to approximately 6.626176 x 10-34 joule-seconds. In the centimeter-gram-second (cgs) or small-unit metric system, it is equal to approximately 6.626176 x 10-27 erg-seconds.
The energy E contained in a photon, which represents the smallest possible 'packet' of energy in an electromagnetic wave, is directly proportional to the frequency f according to the following equation:
E = hf
If E is given in joules and f is given in Hertz (the unit measure of frequency), then:
E = (6.626176 x 10-34) f
and conversely:
f = E / (6.626176 x 10-34)